Thirona receives FDA clearance for LungQ 4 software
In navigational bronchoscopy, this can support the treatment of lung cancer, emphysema, and chronic airway diseases.
Published on October 18, 2025
Team IO+ selects and features the most important news stories on innovation and technology, carefully curated by our editors.
Thirona, a global leader specializing in advanced analysis of thoracic CT images using artificial intelligence, has received U.S. Food and Drug Administration (FDA) clearance for LungQ® 4, the latest version of its AI-powered CT analysis software.
This new clearance builds on version 3 of LungQ®, cleared by the FDA last year, which introduced advanced lung segmentation capabilities, a critical foundation for accurate regional-level analysis. LungQ® 4 extends these capabilities into:
• Enhanced peripheral airway segmentation.
• Estimated chronic perfusion defects from CT.
• Post-treatment detection of endobronchial implants.
The FDA clearance of LungQ® 4 underscores Thirona’s commitment to continuous innovation and performance leadership in AI-driven lung analysis. Since its inception in 2014, Thirona has remained focused on realizing its mission to translate validated AI technology into practical, clinically reliable tools designed to enhance pulmonary intervention planning and guidance, and to accelerate respiratory clinical trials evaluating treatment efficacy.
“Each new FDA clearance is a great confirmation of our uncompromised focus on quality and performance, continuously improving our software to make a real impact in clinical practice,” said Eva van Rikxoort, PhD, CEO of Thirona. “With LungQ® 4, we’ve come a long way in translating our AI technology into trusted tools that actually work in everyday care, implemented in close collaboration with our partners across advanced clinical systems and solutions used by physicians around the world."
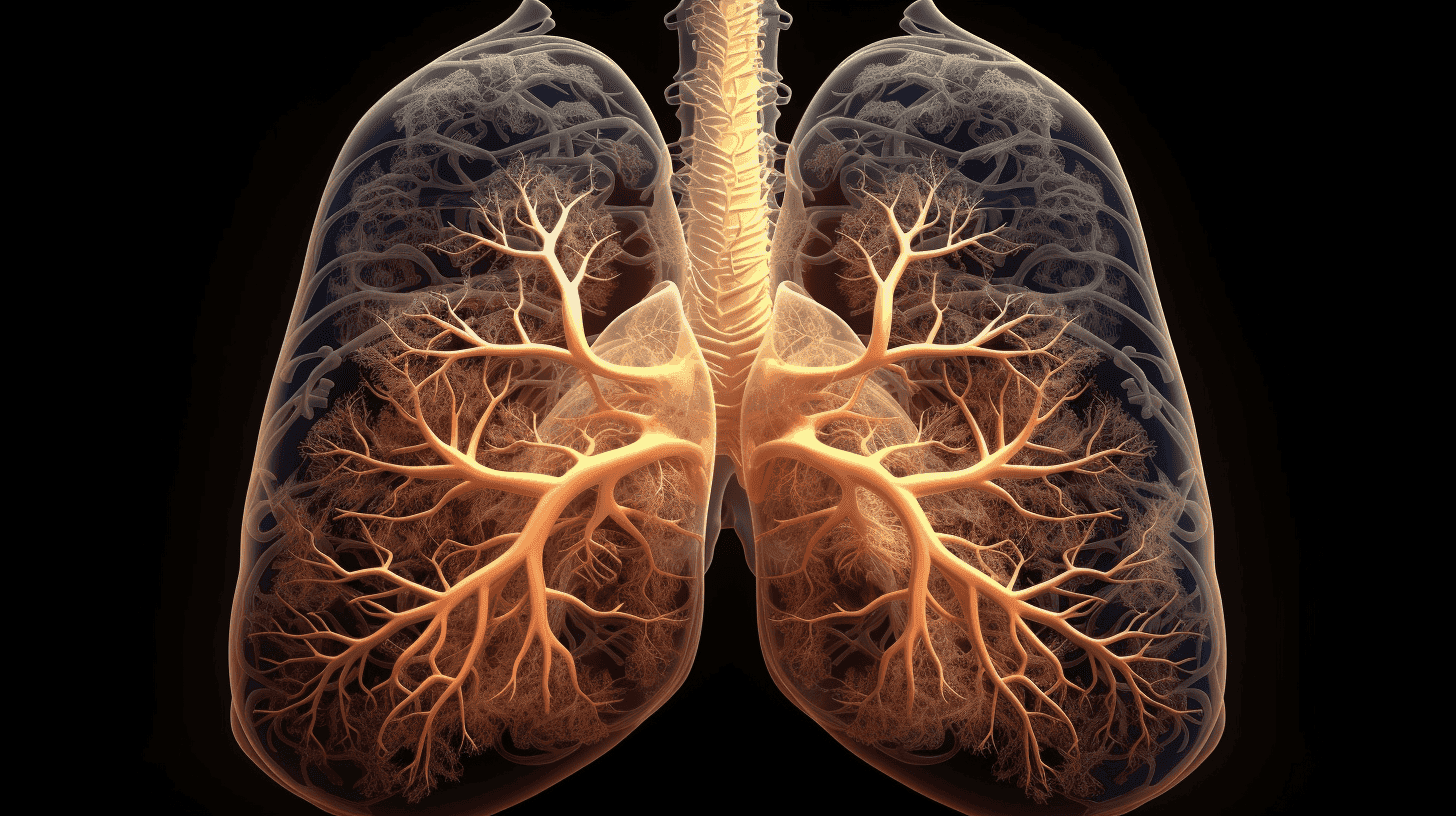
Enhanced Peripheral Airway Segmentation
Leveraging Thirona’s FDA-cleared and CE-certified segmentation framework, along with advanced deep learning models, LungQ® 4 provides enhanced airway segmentation analysis, enabling identification of distal airways within previously inaccessible peripheral regions of the lung. By combining this extended analysis with anatomical airway mapping, LungQ® 4 delivers a detailed anatomical context that enhances clinical interpretation.
In navigational bronchoscopy, this can support precise access planning for distal airway targets in bronchoscopic interventions for the treatment of lung cancer, emphysema, and chronic airway diseases. In respiratory disease assessment, it can provide consistent anatomical referencing to visualize airway abnormalities up to the peripheral bronchial branches, enabling a clearer structural understanding of disease patterns within the bronchial tree.
Already available for clinical use in Europe, the UK, and Australia, this FDA clearance of PXT opens new opportunities for future integration of the analysis into precision-driven interventional workflows in U.S. hospitals.
