Growing tissues, fighting tumors: the Accardo lab’s mission
The Delft University of Technology lab is making strides in studying brain tissue culture.
Published on September 4, 2025

© Delft University of Technology
Mauro swapped Sardinia for Eindhoven and has been an IO+ editor for 3 years. As a GREEN+ expert, he covers the energy transition with data-driven stories.
“Passion, patience, and perseverance are the main skills that are required to work in my lab,” says Professor Angelo Accardo. The scientist is the head of a lab in the Precision and Microsystems Engineering (PME) department of Delft University of Technology (TU Delft)’s Faculty of Mechanical Engineering. Undoubtedly, having all three Ps is essential to researching the most fascinating yet mysterious of the human body: the brain.
The Accardo lab specializes in creating engineered scaffolds, microenvironments that enable human tissues to grow, just as they do in nature. By analyzing these environments, researchers can gain a deeper understanding of how brain cells function, thereby opening up new possibilities for disease treatment.
A pioneering brain cancer treatment
One of the research projects the lab is working on, in collaboration with the Leiden University Medical Center and the Holland Proton Therapy Center, focuses on the use of proton therapy to treat glioblastoma. As the most lethal form of brain cancer, it starts in astrocytes, a type of glial cell in the brain and spinal cord. The tumor cells multiply, spreading into other areas of the nervous system. Like other glial cells, astrocytes play a vital role in ensuring the function of nerve cells.
Proton therapy is a promising option to treat glioblastoma, being more precise in targeting cancer cells. This technique also reduces collateral damage to healthy tissues compared to conventional X-ray radiotherapy, one of the most commonly used treatment options currently. Accardo’s lab is creating 3D models that enable the formation of networks where brain cancer cells cluster and proliferate in vivo. The models are then treated with proton therapy to assess tissue response to the radiation.
One way to fabricate these structures is the so-called two-photon polymerization (2PP) technique, which essentially applies laser beams to solidify liquid biomaterials. “By moving a laser beam, we can create 3D microstructures with a resolution that goes down to 200 nanometers, about 1000 times thinner than the diameter of human hair,” explains Accardo.
These artificial models proved to be close to the natural ones, also significantly outperforming in vitro models—structures replicated on a flat laboratory slide. “We have seen that the DNA damage proton therapy inflicts on our 3D models is lower than that of their 2D counterparts,” underlines Accardo. “This is what we were hoping for, as previous studies on actual brain cancer tumors have shown the same evidence. Therefore, these 3D models reproduce better what is happening in the brain, and we hope to use them as a future benchmark tool for patients.”
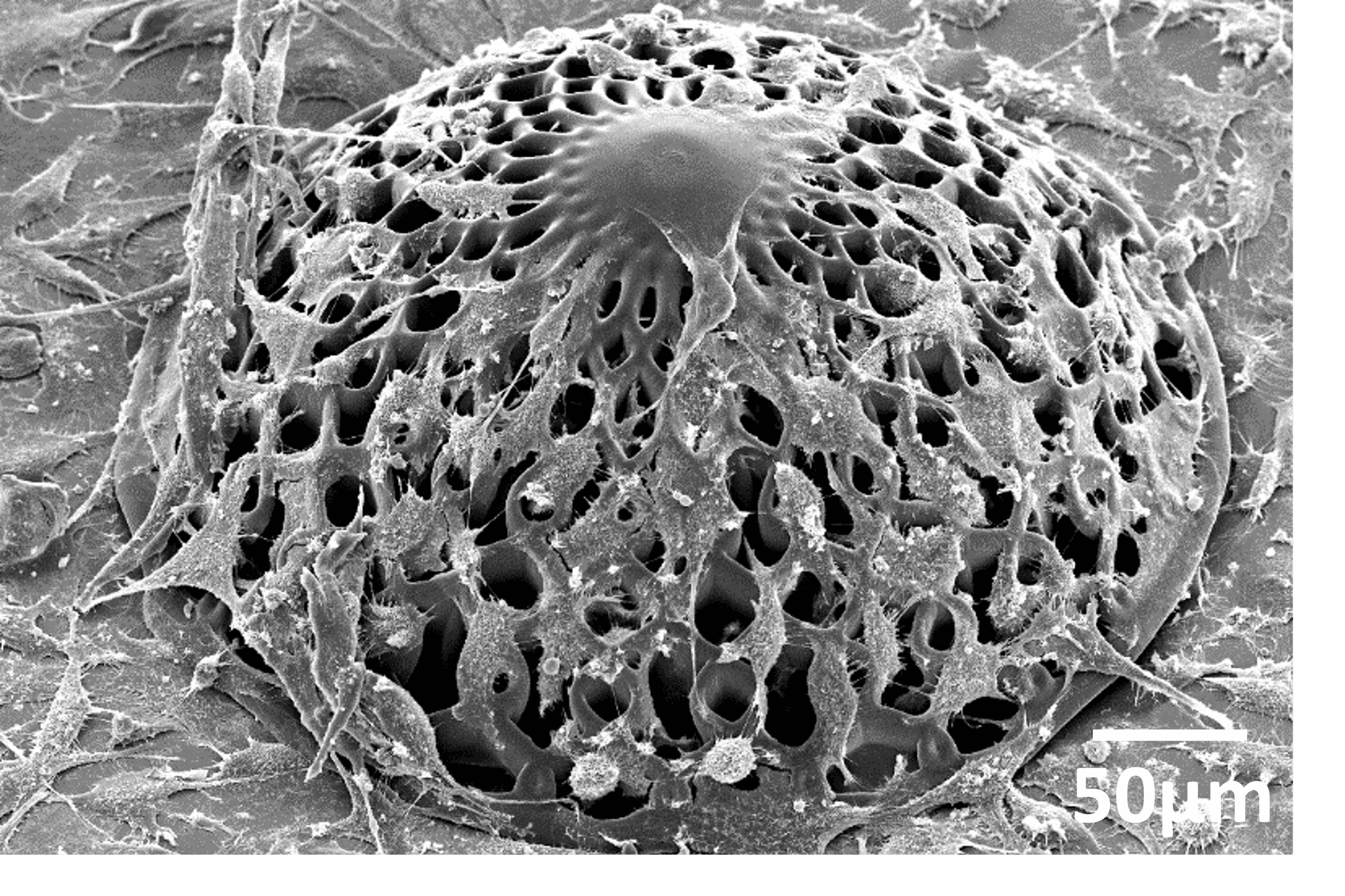
A scaffold designed for glioblastoma research - © Accardo Lab
Studying the correlation between autism and a rare genetic disease
A dozen people, including PhDs, postdocs, and master’s students, are part of the Accardo lab. Among them is Azza Jacobs, a biomedical engineering master’s student. She developed engineered cell micro-environments in the context of a larger project on brain organoids, autism spectrum disorder, and its possible genetic connection to Tuberous Sclerosis Complex (TSC). A rare genetic disease, TSC causes non-cancerous tumors or lesions in the brain and other parts of the body. Previous research has shown that people with autism spectrum disorder suffer from TSC, making it a potentially early marker to diagnose autism before symptoms arise.
However, getting a child’s brain tissue is a very invasive operation. “What we do instead is to use induced pluripotent stem cells, obtained originally from reprogrammed skin cells of the patients. Then, we culture them within engineered scaffolds to become brain organoids,” explains Jacobs. Organoids are structures derived from tissue culture. However, the conventional scaffold-free growth of this tissue culture is often uncontrolled and varies from batch to batch. Furthermore, the inner parts of these structures often clump together, lacking vascularization and ultimately dying.
.jpg)
Azza Jacobs
Master's student at TU Delft
She developed engineered cell micro-environments in the context of a larger project on brain organoids, autism spectrum disorder, and its possible genetic connection to Tuberous Sclerosis Complex (TSC).
As a remedy to these functional problems, Jacobs is using micro-digital light processing (µ-DLP), another 3D printing technique, to create scaffolds where these cultures can grow in a controlled manner. These porous hydrogel structures mimic the mechanical properties of brain tissues. “This way the cells can easily grow inside the scaffold, proliferating, and simulating the behavior of human brain cells,” Jacobs underlines.
Culturing and comparing both healthy and TSC-affected brain cells takes several weeks to grow a mature structure. The goal is to study their behavior, morphology, and gene expression, ultimately paving the way for potential treatment options. This project was conducted in collaboration with the Amsterdam University Medical Center.
Bone tissue culture
Another interest of the Accardo lab is the application of these techniques to bone tissue culture. Drawing full inspiration from nature, a significant difference between bone tissues and brain tissues is the higher stiffness of bone tissues, which is approximately six orders of magnitude more rigid than that of neural tissues. There is no shortage of ambition in this strand of research either.
“For instance, we are working in collaboration with the Department of Biomechanical Engineering on meta-biomaterials designs for bone tissue engineering. Particularly, scaffolds with such properties can have a direct effect on the mechanobiology of bone cells. This aspect plays a fundamental role in prospective use as implants, such as total hip replacement, where the contact between the host tissue and the implant must be optimized to prevent infections,” explains the professor.
Serving patients
The three Ps are certainly shaping the vibe of the research group, with Accardo being proud of the work done by his group and by the continuous influx of ideas and projects. Ultimately, the goal is to deliver the results of all these efforts to those who need them most: patients.
“What I dream of is that, in ten years, we can utilize our approach to treat glioblastoma to perform minimally invasive biopsies in a patient's tumor. Take those cells, culture them in our environment, assess the dose of proton therapy that yields the best results, and use this as a benchmark tool to apply directly to the patient,” he concludes.
